
ClearStrand-ASD plus Autism Diagnostic Evaluation
A diagnostic evaluation for autism may provide you with the peace of mind you deserve.

Autism Diagnostic Evaluation for ClearStrand-ASD Patients
Children aged 1-48 months may now receive ClearStrand-ASD + an autism diagnostic evaluation by an independent autism specialist.
If your child is diagnosed with autism, it will provide you with a diagnostic report that is necessary to access autism-specific therapy they need.

How it works
- 1 Fill out the Get Started form, meet with a Customer Care representative to discuss next steps, and request ClearStrand-ASD + Virtual Diagnostic Evaluation
- 2 Complete ClearStrand-ASD testing
- 3 Receive referral to complete the virtual autism diagnostic evaluation with one of our partners
Your child must live in an eligible state and be 16-48 months at the time of the evaluation. If they are not yet 16 months, they will receive a referral once they are 16 months. Testing must be completed within 90 days of the referral.
Get Started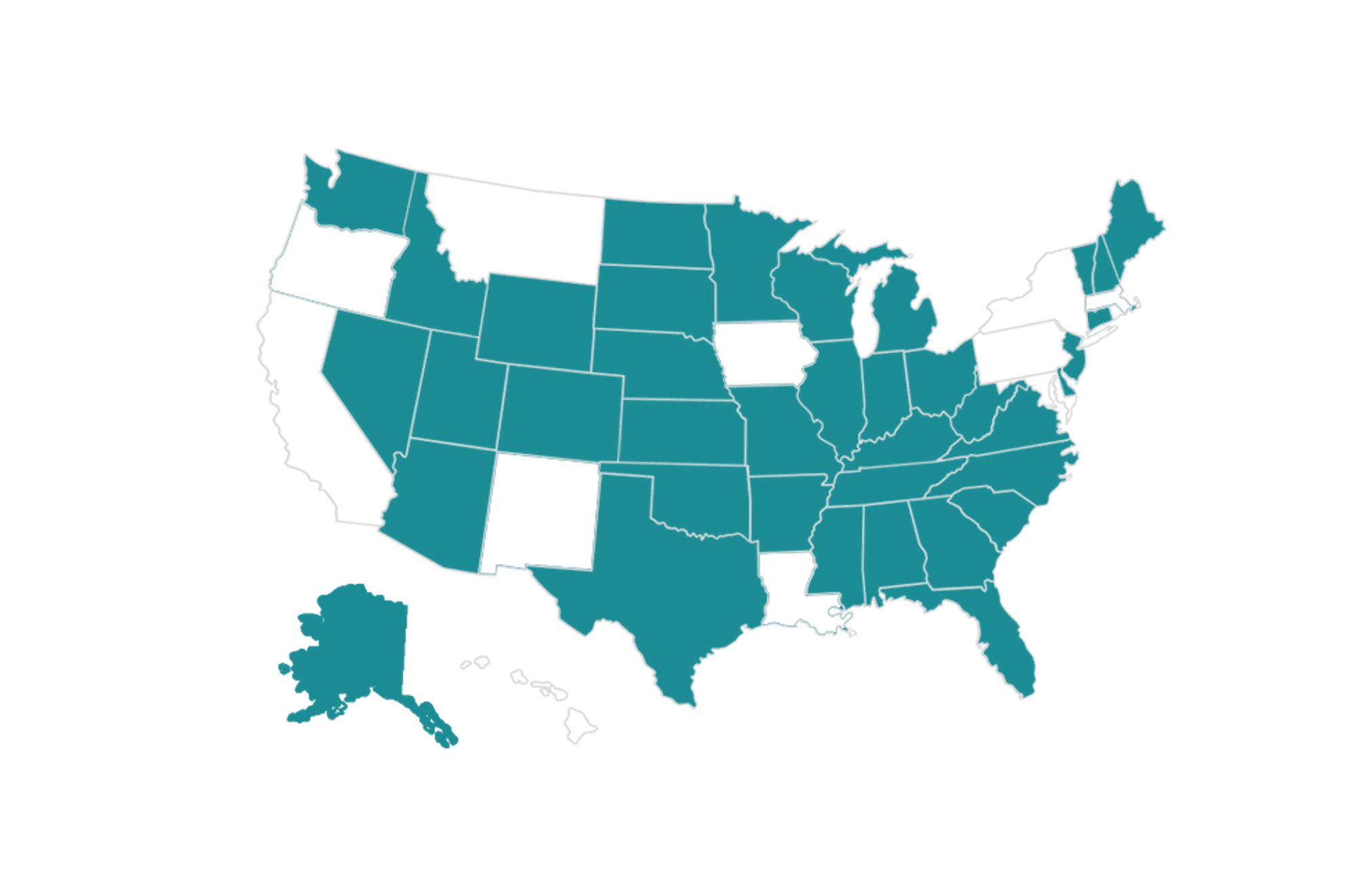
Now available in 42 states + DC for $2,500
Clearstrand-ASD + Virtual Dx is available for patients in all states except for those in California, Hawaii, Iowa, Louisiana, Massachusetts, Montana, New Mexico, and New York.
The price includes the ordering of ClearStrand-ASD through a telehealth provider, kit shipment, testing of the sample at our CLIA-certified lab, a post-test virtual visit to review the ClearStrand-ASD results, and a virtual diagnostic evaluation with an autism specialist.
To be notified when it is available in your state, fill out our contact form.

If your child is under 16 months of age
This autism diagnostic evaluation is valid for children aged 16 - 48 months. If your child gets ClearStrand-ASD testing before 16 months of age, they will still be eligible for the autism evaluation when they turn 16 months.
In the meantime, there are many resources available to better understand developmental milestones and options to intervene early. Your state’s early intervention program may be a good place to start.

Give your child the advantage of objective insights
Take a proactive step in supporting your child’s development and well-being with ClearStrand-ASD.
Page contents
Important information
ClearStrand-ASD is a biochemical test intended to help health care providers rule out autism spectrum disorder (ASD) when it is a concern in children aged 1 month up to 4 years (48 months) of age. It detects a biomarker associated with autism using a strand of hair. ClearStrand-ASD must be ordered by a licensed health care provider. Rx only. ClearStrand-ASD results can be negative or non-negative.
A child with a negative result is unlikely to be on the autism spectrum. A child with a non-negative result may need further autism diagnostic evaluation. ClearStrand-ASD is not a standalone diagnostic test. Health care providers should consider the test result in the context of other factors relevant to their clinical decision making. ClearStrand-ASD analyzes a strand of hair to map the dynamic patterns of an individual's unique biological response to environmental exposures over time at a molecular level and uses an algorithm to assess the likelihood of autism from the patterns. It is not a genetic test.
The test is performed by the LinusBio CLIA-certified laboratory (CLIA #31d2307499) and has not been cleared or approved by the US Food and Drug Administration (FDA).
